Here is a mash-up of several “Nature” magazine reports and daily bulletins, plus a few Zoom chats over the weekend, and a few bits from some pre-prints
25 January 2021 (Chania, Crete) – Moderna announced today that its COVID-19 vaccine protects against British and South African variants, but also said the company has begun developing a new vaccine against the South African variant of COVID as a precaution. The finding was largely encouraging but contained a clear warning sign: even though the vaccine generated disease-fighting antibodies that worked against the South African variant, that efficacy was diminished. Moderna said there was a reduction in response, prompting the company to design a new potential vaccine that could be added on to the current two-dose regimen.
As concern grows over faster-spreading variants of the SARS-CoV-2 coronavirus, laboratories worldwide are racing to unpick the biology of these viruses. Scientists want to understand why variants identified in the United Kingdom and South Africa seem to be spreading so quickly, with higher lethality, and whether they might diminish the potency of vaccines or overcome natural immunity and lead to a spate of reinfections. Said Jeremy Luban, a virologist at the University of Massachusetts Medical School in Worcester, MA in a Zoom chat over the weekend:
“Many of us are scrambling to make sense of the new variants, and the million-dollar question is what significance this will have for the effectiveness of vaccines that are currently being administered”.
The first lab results are trickling in and many more are expected in the coming days, as researchers rush to probe the viral variants and their constituent mutations in cell and animal models of SARS-CoV-2, and test them against antibodies elicited by vaccines and natural infections. One preprint noted that a mutation shared by both variants did not alter the activity of antibodies produced by people who received a vaccine developed by Pfizer and BioNTech. Said Vineet Menachery, a virologist at the University of Texas Medical Branch in Galveston, TX whose team is studying the variants:
“By the end of this month we’ll have much more information”.
Underlying biology
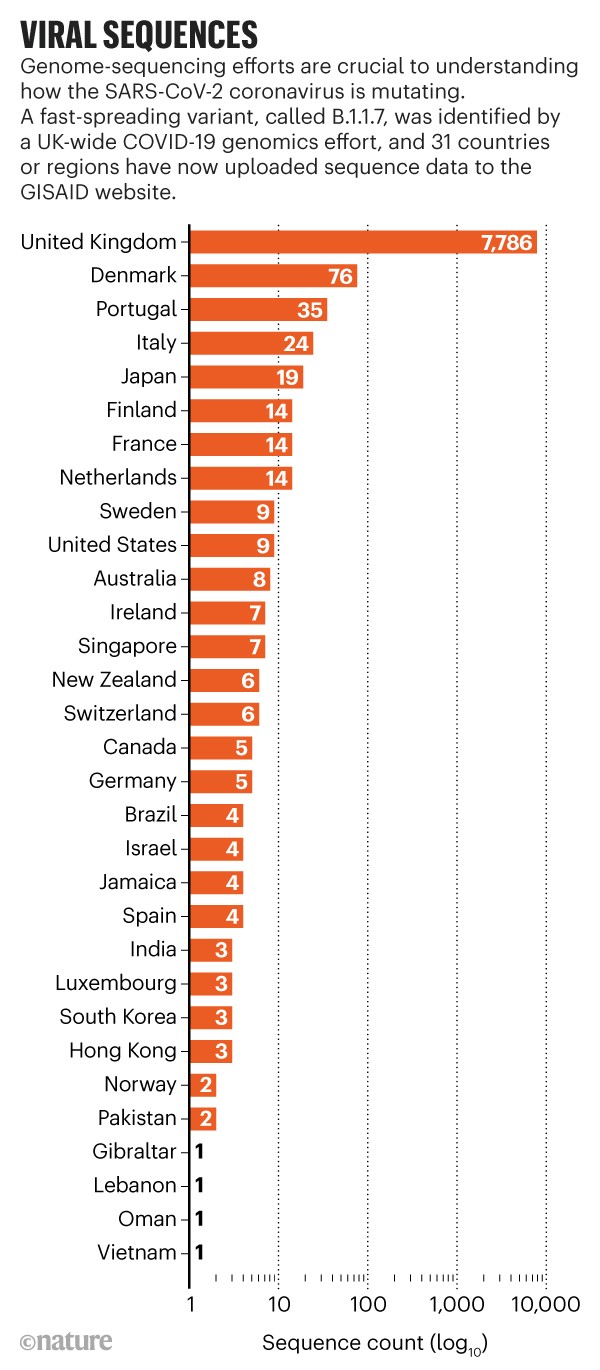
Researchers spotted the coronavirus variants in late November and early December 2020 through genome sequencing. A UK-wide COVID-19 genomics effort determined that a virus variant now known as B.1.1.7 had been behind surging case numbers in the southeast of England and London; the variant has now spread to the rest of Britain and has been detected in tens of countries worldwide.
Also, a team led by bioinformatician Tulio de Oliveira at the University of KwaZulu-Natal in Durban, South Africa, connected a fast-growing epidemic in the country’s Eastern Cape Province to a coronavirus variant that the researchers call 501Y.V2. The UK and South African variants emerged independently, but both carry a bevy of mutations – some of them similar – in the coronavirus spike protein, through which the virus identifies and infects host cells, and which serves as the chief target of our immune response.
Epidemiologists studying the growth of the B.1.1.7 variant in the United Kingdom have estimated that it is around 50% more transmissible than other variants of SARS-CoV-2 known to be in circulation – an insight that contributed to the UK government’s decision to enter a third national lockdown on 5 January. Wendy Barclay, a virologist at Imperial College London and a member of a group advising the UK government on its response to B.1.1.7, said “the epidemiology has really shown us the way here”.
But, she added, scientists must determine the underlying biology. Understanding what properties of the virus make it more transmissible allows governments to be more informed about policy decisions.
One challenge is disentangling the effects of the mutations that distinguish the UK and South African lineages from their close relatives. The B.1.1.7 variant carries eight changes that affect the spike protein, and several more in other genes; samples of the South African 501Y.V2 variant carry up to nine changes to the spike protein. Working out which are responsible for the rapid spread of the variants and their other properties is an enormous challenge. No one thinks there’s a single mutation that’s accounting for all of it.
Much of the effort is focused on a change to the spike protein that is shared by both lineages, called N501Y. This mutation alters a portion of the spike, called the receptor binding domain, that locks onto a human protein to allow infection. One hypothesis that previous studies have hinted at is that the N501Y change allows the virus to attach to cells more strongly, making infection easier.
The N501Y mutation is one of several that teams are testing in hamsters, which are models for studying SARS-CoV-2 transmission. One team had reported last year that a different mutation to the spike protein enabled viruses to reach greater concentrations in the upper airways of hamsters, compared with viruses lacking the change. That’s what many are expecting with these mutations. If that’s the case, that’s going to be driving their transmissibility. A pre-print published in late December supports that hypothesis: it found more SARS-CoV-2 genetic material in the swabs of people infected with the B.1.1.7 variant, than in those from individuals infected with viruses lacking the N501Y change.
Antibody tests
The rapid spread of the variants has triggered efforts to contain their spread, through lockdowns, border restrictions and heightened surveillance. Adding to the sense of urgency is the worry that the variants could weaken immune responses triggered by vaccines and previous infection. Both variants harbour mutations in regions of the spike protein that are recognized by potent “neutralizing”, or virus-blocking, antibodies. This raises the possibility that antibodies to these regions – the receptor binding domain and a portion called the N-terminal domain – could be affected by the mutations.
As a result, academic and government researchers and vaccine developers are now working around the clock to address the question. All participants in the Zoom calls noted “we must move at crazy speeds”.
In one experiment, a team found that the 501Y mutation, at least, did not drastically affect the activity of neutralizing antibodies in convalescent serum – the antibody-containing portion of blood taken from people who have recovered from COVID infection. This suggests that the 501Y mutation is unlikely to alter immunity.
Other mutations, however, might affect immunity. Chief among those is another receptor-binding-domain mutation, called E484K, that one team has identified in the 501Y.V2 variant. The team is working with African health research organizations to test the variant against convalescent serum and serum from people who have been vaccinated in trials.
Immune escape
There is emerging evidence that the E484K mutation can enable the virus to escape some people’s immune responses. As I noted in an earlier post, last month a team at the Toscana Life Sciences Foundation in Siena, Italy, grew SARS-CoV-2 in the presence of low levels of one person’s convalescent serum. The goal was to select for viral mutations that evade the diverse repertoire of antibodies generated in response to infection. The experiment wasn’t necessarily supposed to work. But within 90 days, the virus had picked up 3 mutations that made it impervious to the person’s serum: one of these was the E484K mutation found in the South African variant; the others were N-terminal domain changes found in both the South African and the UK variant. The team said that was surprising because it suggested that the individual’s entire antibody response against SARS-CoV-2 was directed against a small portion of the spike protein.
The lab-evolved strain proved less resistant to convalescent sera from other people. But the experiment suggests that mutations such as E484K and N-terminal domain changes that are carried by both variants could affect how antibodies generated by vaccines and previous infection recognize the mutations.
A more pressing question is whether such changes will alter the real-world effectiveness of vaccines. In a preprint last week one team reported that E484K and several other mutations can escape recognition by antibodies in peoples’ convalescent sera to varying degrees.
Many scientists remain “hopeful” that the mutations in the variants won’t substantially weaken the performance of vaccines. The shots tend to elicit whopping levels of neutralizing antibodies, so a small drop in their potency against the variants might not matter. Other arms of the immune response – T-cells, for example – that are triggered by vaccines might not be affected.
But these new tests have underscored the need to track changes in the virus and prepare for the eventuality that, like flu shots, a coronavirus vaccine might need to be updated and administered on a regular basis. All bets are off – for now – if the current vaccines are going to remain effective for the things that really count: keeping people from getting deathly ill.